Artificial molecules that mimic DNA
Not only can synthetic molecules imitate the structures of their biological counterparts, they can also assume their functions and even outcompete them. This has recently been demonstrated by researchers from the CNRS, Inserm and Bordeaux University, who have developed an artificial sequence mimicking the surface features of DNA for the first time. This artificial molecule is able to inhibit the activity of several DNA-binding enzymes, including the one used by HIV to insert its genome into that of its host cell. These results, published on April 2, 2018 in Nature Chemistry, pave the way for new pharmacological tools based on inhibiting DNA–protein interactions.
DNA, the central molecule of life, carries genetic information organized in the two complementary strands of its double helix. In order for this genetic information to be read and executed, or on the contrary to prevent or regulate its expression, a number of proteins interact with DNA, for example by “binding” to the negative charges located on its surface. This is the case for HIV integrase, which enables the insertion of the viral DNA into human DNA and topoisomerase, an enzyme that acts to release tensions within the DNA molecule when it is supercoiled.
Researchers at the Institut de chimie et biologie des membranes et nano-objets (CNRS/Bordeaux University/Bordeaux INP)1
, the Laboratoire de microbiologie fondamentale et pathogénicité (CNRS/Bordeaux University) and the Institut de recherche en cancérologie de Montpellier (Inserm/Montpellier University) have successfully synthesized helical molecules that precisely imitate the surface features of DNA's double helix and notably the position of its negative charges. These molecules are derived from aromatic foldamers, synthetic objects with a strong propensity to adopt folded conformations, in this case, a single helix. The imitation is so convincing that these foldamers trick certain proteins that would normally bind to DNA, including topoisomerase and HIV integrase. The researchers demonstrate that the synthetic mimics make better ligands for these enzymes than natural DNA, even at weak concentrations of foldamers. It seems that this efficacy is due to subtle differences between their structure and that of natural DNA.
These DNA mimics pave the way for as yet unexplored approaches to inhibiting DNA–protein interactions, which could, in the future, lead to new medicines.
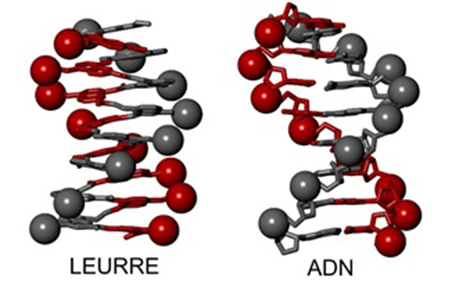
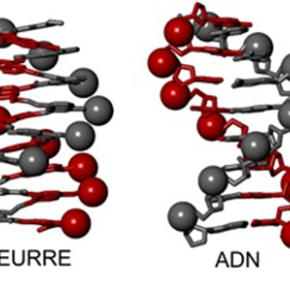
Representation of a DNA double helix (right) and a DNA mimic (left). The mimic comprises a single helix, on the surface of which two networks of negative charges (red and grey spheres) are positioned like the negative charges in the two strands of DNA.
- 1 Including researchers from Ivan Huc's team at the Institut européen de chimie et biologie (CNRS/Inserm/Bordeaux University).
Single helically folded aromatic oligoamides that mimic the charge surface of double-stranded B-DNA. Krzysztof Ziach, Céline Chollet, Vincent Parissi, Panchami Prabhakaran, Mathieu Marchivie, Valentina Corvaglia, Partha Pratim Bose, Katta Laxmi-Reddy, Frédéric Godde, Jean-Marie Schmitter, Stéphane Chaignepain, Philippe Pourquier, and Ivan Huc. Nature Chemistry, April 2, 2018. DOI: View web site