Magnetic cellular "Legos" for the regenerative medicine of the future
By incorporating magnetic nanoparticles in cells and developing a system using miniaturized magnets, researchers at the Laboratoire Matière et Systèmes Complexes (CNRS/Université Paris Diderot), in collaboration with the Laboratoire Adaptation Biologique et Vieillissement (CNRS/UPMC) and the Centre de Recherche Cardiovasculaire de Paris (Inserm/Université Paris Descartes), have succeeded in creating cellular magnetic “Legos.” They were able to aggregate cells using only magnets and without an external supporting matrix, with the cells then forming a tissue that can be deformed at will. This approach, which is detailed in Nature Communications on September 12, 2017, could prove to be a powerful tool for biophysical studies, as well as the regenerative medicine of tomorrow.
Nanotechnology has quickly swept across the medical field by proposing sometimes unprecedented solutions at the furthest limits of current treatments, thereby becoming central to diagnosis and therapy, notably for the regeneration of tissue. A current challenge for regenerative medicine is to create a cohesive and organized cellular assembly without using an external supporting matrix. This is a particularly substantial challenge when it involves synthesizing thick and/or large-sized tissue, or when these tissues must be stimulated like their in vivocounterparts (such as cardiac tissue or cartilage) in order to improve their functionality.
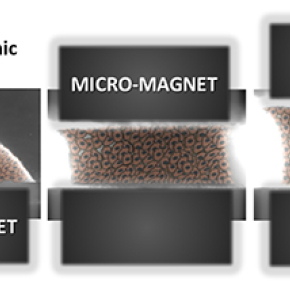
The researchers met this challenge by using magnetism to act on the cells at a distance, in order to assemble, organize, and stimulate them. Cells, which are the building blocks of tissue, are thus magnetized in advance through the incorporation of magnetic nanoparticles, thus becoming true cellular magnetic “Legos” that can be moved and stacked using external magnets. In this new system acting as a magnetic tissue stretcher, the magnetized cells are trapped on a first micromagnet, before a second, mobile magnet traps the aggregate formed by the cells. The movement of the two magnets can stretch or compress the resulting tissue at will.
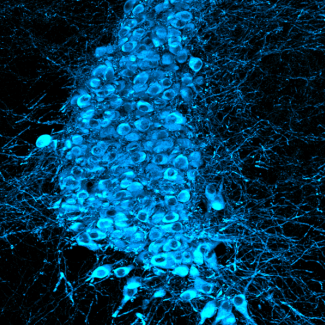
Researchers first used embryonic stem cells to test their system. They began by showing that the incorporation of nanoparticles had no impact on either the functioning of the stem cell or its capacity for differentiation. These functional magnetic stem cells were then tested in the stretcher, in which they remarkably differentiated toward cardiac cell precursors when stimulation imposed “magnetic beating” imitating the contraction of the heart. These results demonstrate the role that purely mechanical factors can play in cell differentiation.
This “all-in-one” approach, which makes it possible to build and manipulate tissue within the same system, could thus prove to be a powerful tool both for biophysical studies and tissue engineering.
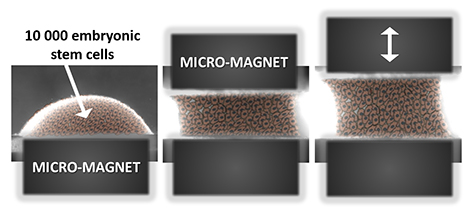
The magnetic stretcher: this all-in-one system can both form and mechanically stimulate an aggregate of embryonic stem cells. The two micromagnets, one of which is mobile, frame the resulting embryonic body, and cyclical stimulation can be adapted to the type of tissue being sought.
A 3D magnetic tissue stretcher for remote mechanical control of embryonic stem cell differentiation. Vicard Du, Nathalie Luciani, Sophie Richard, Gaëtan Mary, Cyprien Gay, François Mazuel, Myriam Reffay, Philippe Menasché, Onnik Agbulut, Claire Wilhelm. Nature communications, 12 September 2017. DOI: 10.1038/s41467-017-00543-2