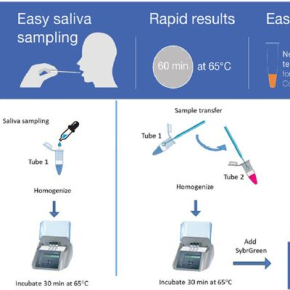
Promising initial results for a COVID-19 diagnostic test in saliva
Scientists in the Sys2diag Laboratory (CNRS/ALCEN) and doctors from University Hospital (CHU) of Montpellier have presented the first results of a clinical trial on the EasyCov SARS-CoV-2 detection test. This shows satisfactory performance for a field test that solves a problem in situations where the RT-PCR test cannot be used. Covering a panel of 123 subjects, these intermediate results were prepublished1 in medRxiv on 30 May 2020.
- 1This work has not yet been validated by peer review.
Started on 11 April 2020 at CHU of Montpellier, the EasyCov clinical trial included 123 people to date and will continue until 180 subjects have been double-blind tested, with the RT-PCR method and with EasyCov. From these intermediate results, this detection test for SARS-CoV-2 developed in the Sys2diag Laboratory (CNRS/ALCEN) has shown few false positives (95.7% specificity) and produced a little under 30% of false negatives (72.7% sensitivity)1 .
Simpler and faster than a RT-PCR test from a nasal swab sample, EasyCov could complement existing screening methods. The RT-LAMP technique on which EasyCov is base amplifies viral RNA then reveals whether it is present in a saliva sample heated for only one hour. The results are read simply by using a coloured reagent.
The double-blind clinical trial has included 93 asymptomatic healthcare workers, among which EasyCov detected one pre-symptomatic case, and 30 infected patients, of whom 10 were recent cases and 20 were at monitoring visits. The subjects were taken in charge and tested at CHU of Montpellier with the RT-PCR method and their saliva was tested with EasyCov in the Sys2diag Laboratory. The results for the two diagnostic tests for this first cohort are now compared in a prepublication on medRxiv.
The clinical trial will continue until about 50 more subjects have been recruited to reach a total of 180. And at the same time , the EasyCov test ought to be available for sale during June 2020, sold by Skillcell (in French only).
© L’Helgouach et al.
- 1A diagnostic test’s specificity is the probability that the test will correctly be negative in those who are not ill and is measured in those without an illness only. Its sensitivity is the probability of correctly detecting patients who do have an illness and is measured in ill patents only.A diagnostic test’s specificity is the probability that the test will correctly be negative in those who are not ill and is measured in those without an illness only. Its sensitivity is the probability of correctly detecting patients who do have an illness and is measured in ill patents only.
EasyCOV:LAMP based rapid detection of SARS‐CoV‐2 in saliva. Nicolas L’Helgouach, Pierre Champigneux, Francisco Santos Schneider, Laurence Molina, Julien Espeut, Mellis Alali, Julie Baptiste, Lise Cardeur, Benjamin Dubuc, Vincent Foulongne, Florence Galtier, Alain Makinson, Grégory Marin, Marie‐Christine Picot, Alexandra Prieux‐Lejeune, Marine Quenot, Francisco Checa Robles, Nicolas Salvetat, Diana Vetter, Jacques Reynes and Franck Molina. Preprint available on medRxiv since May 30, 2020 : https://www.medrxiv.org/content/10.1101/2020.05.30.20117291v1