New materials for storing flammable industrial gases
|
|
An international research team involving the CNRS1 , Air Liquide and the University of Kyoto (Japan) has just demonstrated the very promising capabilities of a new family of materials for storing flammable gases such as acetylene. These materials are nanoporous and flexible and can be modified to improve the adsorption of small molecules at the temperature and pressure conditions required for industrial applications. These results were published on 21 April 2022 in Nature Chemistry.
- 1At the Institut de Recherche de Chimie Paris (CNRS/Chimie ParisTech - PSL).
How do I store more, and better? This summarizes the challenge of transporting flammable gases. To ensure industrial safety, these gases must be handled at defined temperature and pressure conditions that do not allow for optimal storage and release cycles. Existing porous materials can facilitate the capture of certain gases, but their high affinity for these molecules complicates their release: a large amount of gas then remains trapped in the host material.
Scientists have just shown that new patented materials1 could provide a solution, by demonstrating their ability to capture and release acetylene. For a given volume, they can store and release 90 times more acetylene. In that step, it is even possible to recover 77% of the gas stored in a cylinder – far more than with existing porous materials. And all this is at temperature and pressure conditions suitable for industrial applications.
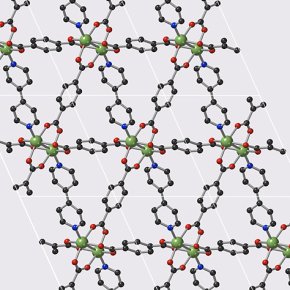
These materials belong to the family of Metal-Organic frameworks (MOFs) that form nanoporous crystal structures. The MOFs studied during this work have the peculiarity of being flexible, and thus offer two states: "open" and "closed", facilitating gas storage and release respectively. In addition, they can be modified to control the storage–release pressure very finely, and thus be suitable for various industrial constraints.
Based on these results, the research team plans to test new modifications to give these flexible MOFs novel properties, for example to facilitate the capture of CO2, methane or hydrogen. Reducing the cost of these new materials remains a major objective in order to develop industrial applications.
This research was carried out as part of the International Research Project2 SMOLAB, which concentrates and reinforces complementary French and Japanese strengths in the field of flexible MOFs and their applications. SMOLAB was created in 2018 by the University of Kyoto and the CNRS, in partnership with avec Air Liquide, Claude Bernard University Lyon 1, Chimie ParisTech / PSL University.
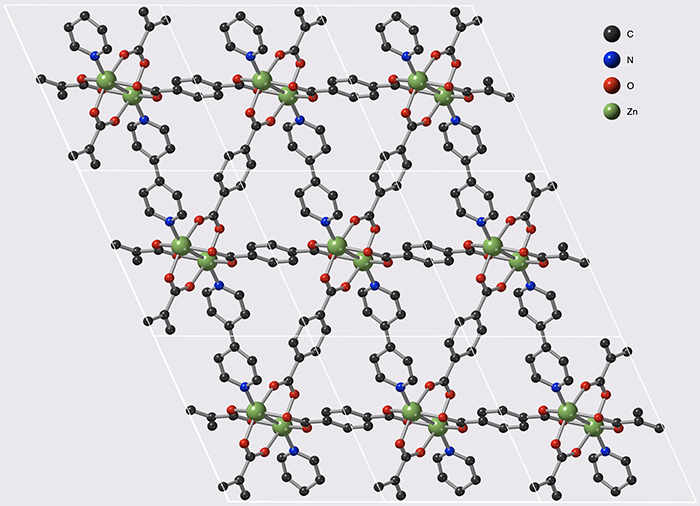
© François-Xavier Coudert/CNRS
- 1Developed by the University of Kyoto and Air Liquide, reference WO2021043492A1.
- 2The International Research Projects are collaborative research projects established between one or more CNRS laboratories and laboratories in one or two foreign countries. Through them established collaborations are consolidated by short- or medium-term scientific partnerships. They include working meetings and seminars, the development of joint research activities including field research, and the supervision of students.
Tunable acetylene sorption by flexible catenated metal-organic frameworks. Mickaele Bonneau, Christophe Lavenn, Jia-Jia Zheng, Alexandre Legrand, Tomofumi Ogawa, Kunihisa Sugimoto, François-Xavier Coudert, Régis Réau, Shigeyoshi Sakaki, Ken-ichi Otake and Susumu Kitagawa. Nature Chemistry, 21 April 2022. DOI:10.1038/s41557-022-00928-x