A technology that "sees" inside commercial batteries
|
|
A multidisciplinary research team involving scientists from the Collège de France, the CNRS, the Université Rennes 1 and the Université de Montpellier has developed a way of monitoring how the evolving chemistry inside a battery, in real time, and throughout its multiple charges and discharges. Presented in Nature Energy on 7 November 2022, this technology paves the way to improve battery performance and design.
Batteries store energy in chemical form: during charge the current forces chemical reactions and the energy is stored, then during discharge a spontaneous electrochemical reaction causes the reverse displacement of electrons in the system. The energy is released to create an electric current.
Controlling and studying the chemistry of batteries is therefore crucial to understanding their operation, and also to improving their design. While this exercise is easy in the laboratory, it is much harder when integrated into a system. However, a multidisciplinary research team1 led by scientists from the Laboratoire Chimie du Solide et de l'Energie (CNRS/Collège de France/Sorbonne Université) has just developed a method to monitor the evolving chemistry of a commercial battery, in real time, while it charges and discharges.
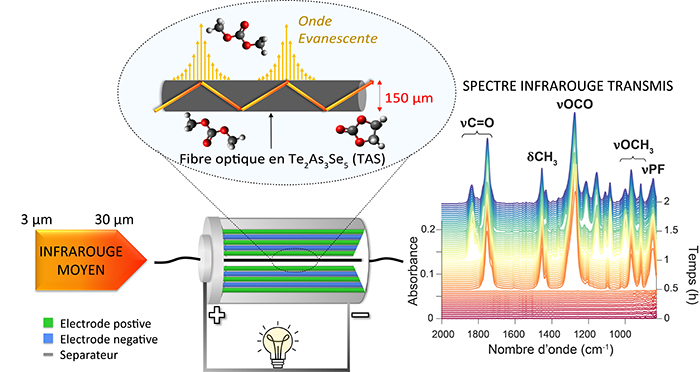
The technology, presented in an article published in Nature, is based on the transmission of infrared light in chalcogenide glass optical fibres placed through a battery. From the interaction of this light with the battery constituents the chemical molecules present around the fibre are identified and tracked.
Researchers were able to observe electrolytes evolution as well as the insertion/extraction of sodium-lithium ions in the electrodes according to the state of charge. And they did that while it was in use, a first! With this system, the scientists were also able to study the interface between the electrolyte and the negative electrode material called the solid electrolyte interphase (SEI). This layer, which is both ion-conducting and electron-insulating, determines how long the batteries last. In particular, the team was able to follow in situ the nature of the chemical species involved in the nucleation and growth of the SEI, which is established during a battery’s very first charge step.
From a practical point of view, these results pave the way for easier and improved battery design. Currently, it takes a long time to optimize electrolytes and protocols to find the best option for an ideal SEI, and thus improve battery the longevity. With this new method, it is possible to quickly and accurately see how each element of the recipe evolves, interacts with others and influences battery performance. The research team continues its work by focusing on the SEI and hopes to be able to reveal all its secrets.
© Gervillié-Mouravieff et al./Collège de France

© Frédérique PLAS / CSE / CNRS Photothèque
- 1In France, this research has also involved teams from the Institut des Sciences Chimiques de Rennes (ISCR; CNRS/École Nationale Supérieure de Chimie de Rennes/Université Rennes 1) and the Institut Charles Gerhardt Montpellier (ICGM; CNRS/École Nationale Supérieure de Chimie de Montpellier/Université de Montpellier). They were carried out as part of the Réseau sur le Stockage Electrochimique de l’Energie.
Unlocking cell chemistry evolution with in-operando fiber optic IR spectroscopy in commercial Na(Li)-ion batteries. Charlotte Gervillié-Mouravieff, Catherine Boussard-Plédel, J. Huang, Cédric Leau, L. Albero Blanquer, Mouna Ben Yahia, Marie-Liesse. Doublet, S. T. Boles, Xianghua H. Zhang, Jean-Luc Adam and Jean-Marie Tarascon. Nature Energy, 7 November 2022. DOI:10.1038/s41560-022-01141-3

